WHO Approve Kenya For Coronavirus experimental Drug Trials

According to a report by International Standard Randomised Controlled Trial (ISRCT), a global registry where all human clinical trials are listed and accredited by WHO, Kenya can now begin recruiting participants for the project dubbed ‘solidarity’.
“There are currently no available vaccines or treatments for Covid-19. Although there have been some suggestions for untested treatments that could be added to the usual care in hospitals, none is known to help.
“The World Health Organization (WHO) is, therefore, organizing a study in many countries in which some of these untested treatments are compared with each other, to discover whether any do help,” ISRCT announced of the initiative.
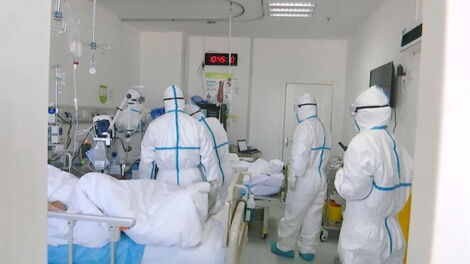
According to the program, the study treatments being considered are remdesivir, chloroquine or hydroxychloroquine, lopinavir plus ritonavir, and interferon-beta, given as daily pills or as daily injections.
It will, however, not be possible for people to volunteer themselves or their relatives to participate in the drug trials.
“The patients who have then been diagnosed with Covid-19 and who have consented to be part of the study will be randomly allocated to receive either local standard care alone or local standard care and one of a list of study drugs.
“During the study, some treatments may get removed from this list, and others may be added to it. Each patient will only receive one of the treatments,” ISRCT further announced.
It also informed that the patients would be followed up for the entire length of their hospital stay and death from any cause would be recorded as the result be used to determine whether a drug is effective.
The length of hospital stay and time to first receiving ventilation (or intensive care) will also be recorded and used to determine the drug’s effectiveness.
The ISRCT informed that the final results of the trials would be published in March 2021, but the protocol indicates periodic updates will be available, with the option of coordinators making adjustments when necessary.
According to the report, Kenya is one of the 23 countries set to benefit from the initiative.
The others are Argentina, Brazil, Canada, Germany, Honduras, India, Indonesia, Iran, Ireland, Israel, Italy, Lebanon, Malaysia, Norway, Peru, Philippines, Qatar, Saudi Arabia, South Africa, Spain, Switzerland and Thailand.
By JOHN PAUL SIMIYU
Source-kenyans.co.ke









It is disappointing to see Kenya has been approved for the drug trials. There only about 400 cases of Covid 19 in Kenya and over 1.2 million in the USA. Are Kenyan lives less valuable than of those in the US A?